Welcome
Welcome to the homepage of the EACPT Education Working Group. Prescribing medicines is a fundamental skill for most doctors. Medicines are the commonest form of therapeutic intervention to improve health but also the commonest cause of iatrogenic disease. Safe and effective use of medicines should be underpinned by a good education in the principles of clinical pharmacology and therapeutics, both within medical schools and during postgraduate studies. The stated aims of EACPT within its statutes include ‘improving and harmonizing the teaching of the rational use of drugs at both undergraduate and postgraduate levels’ and ‘promoting high professional standards in the prescribing of drugs’.
Colleagues who would like to contribute to the work of the Working group should contact the secretary Jelle Tichelaar j.tichelaar@amsterdamumc.nl
Mission
The aim of the EACPT Education Working Group (EWG) is to support the improvement of undergraduate and postgraduate CPT teaching in Europe and, in this way, contribute to an increase in the number of effective, safe and efficient prescribers, and the number of high quality CPT teachers and researchers. The EWG tries to achieve this aim by:
-
organizing meetings on CPT education
-
organizing training courses and developing materials for CPT Teachers
-
developing and supporting research on CPT education
-
creating a network of European Teachers in Therapeutics (NETT)
-
hosting a CPT education website
-
granting Education Awards and Certification
Membership
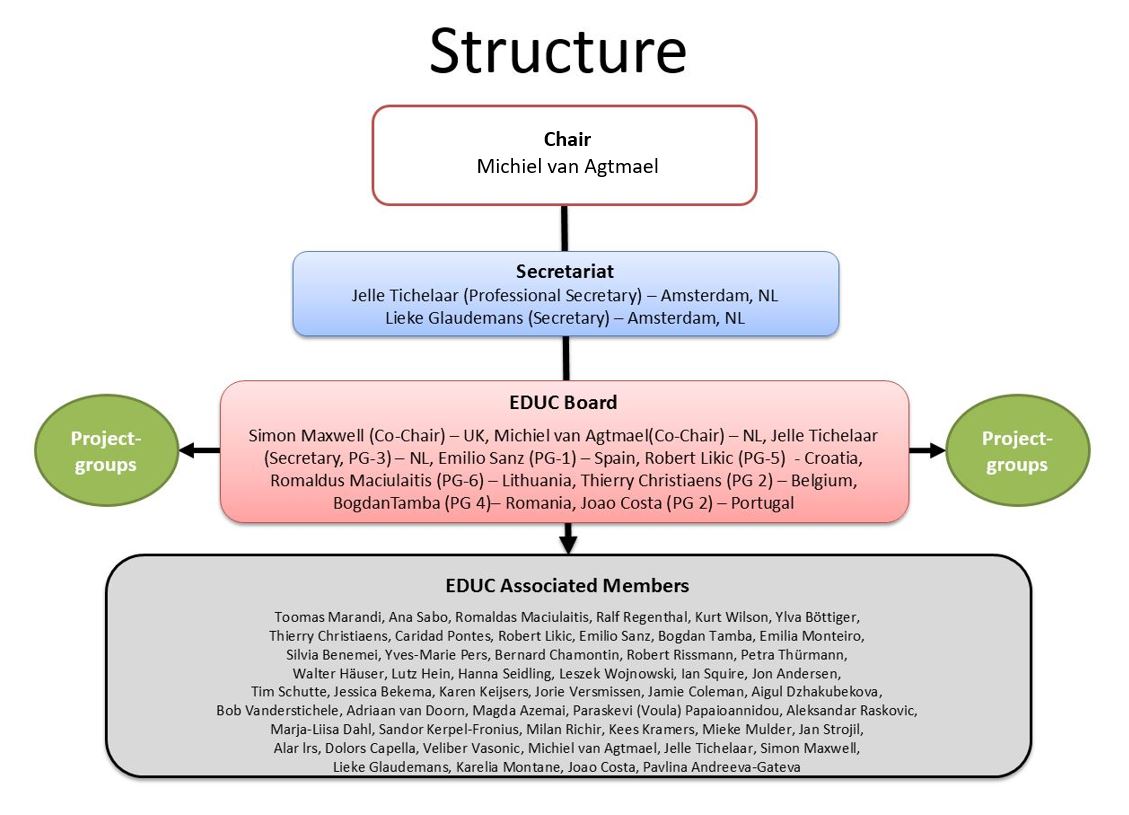
The EACPT Education working group (EWG) consists of board members and associate members.
The EWG board is responsible for reaching the aims (see above) and the various EWG projects that would lead to reaching these aims (see below). The board consists of a chair, a secretary and 7 members. They meet ones a year during a face tot face meeting (Amsterdam or at congress venue), and 3 times by Zoom. EWG board members may create a working group in order to carry out one or more projects.
One co-chairman is a co-opted member of the EACPT Executed Committee in order to tune the policies of both committees.
The EWG associate members advice the EWG board, participate in one or more EWG projects and/or may join the (Linked In) Network of CPT Teachers (see below). They meet every two years during the EACPT congress.
Every two years, during the EACPT congress, elections take place for the EWG board (see statutes below). Regularly the European National CPT Societies are requested to nominate suitable EWG members.
The secretariat of the EWG is currently situated in Amsterdam (VU University Medical Center)
Statutes
1 Nomination EWG members (board and associate members)
1.1 EACPT will ask all associated countries to confirm or amend their EWG National Representatives 6 months before each EACPT congress. These representatives will then be confirmed as EWG board member or EWG associate member subsequently prior to each EACPT Congress.
1.2 Nominating an EWG National representative can be done by e-mail and should include:
(i) the nominee’s name, position and e-mail address,
(ii) a brief reason why they should be considered for membership including, ideally, a description of important educational achievements to date, and
(ii) a simple one or two page biography
1.3 The nominee should have agreed to be nominated!
1.4 Nominations can be sent to the EWG office (awm.glaudemans@vumc.nl).
2. Election EWG board members, co-chair and secretary
2.1 The EWG Board will be composed of those National Representatives or EWG associate members who are making a demonstrable contribution to the Working Groups. The EWG Board membership will be kept under annual review and new nominations ratified by the EWG Board.
2.2 Co-Chairs will be elected from within the EACPT Board with any member able to self-nominate for the position. EACPT Executive Committee will be able to make a nomination and must ratify the subsequent selection at the EACPT congress;
2.3 Co-Chairs will be appointed for 4 years with the opportunity to be re-elected for a second term if this is supported by the EWG Board. Co-Chair periods of office will overlap by 2 years.
2.4 The Professional Secretary will be elected by the EWG Board. Their period of office will be flexible.
– Current members
EWG Board:
Since 1994, Michiel does academic work (AMC, Erasmus MC, since 2001 VUmc) with a mix of clinical work, teaching and research. He wrote a PhD thesis in 1999, on Clinical & pharmacological studies of artemether in the treatment of malaria. He has a long clinical experience in the care for patients with infectious diseases. Since 2013, he is the head of the section Pharmacotherapy with a focus on education and research in education. He is a Professor in Clinical pharmacology training and education.
Research interests: Therapeutic reasoning, how to teach pharmacotherapy to students and doctors with a focus on antimicrobial therapy and stewardship. He believes in student-led education. His mission is to improve rational and safe prescribing in (future) doctors.
Publications
please click link : agtmael m – Search Results – PubMed (nih.gov)
Dr Likic qualified in Medicine with honors in Zagreb in 2001, after receiving the Dean’s award for best student of medicine in 1999. Following internship, he undertook training in general internal medicine at the University Hospital Zagreb, while at the same time researching the effectiveness of problem based learning applied to the teaching of clinical pharmacology which both led to him getting a board certification in internal medicine in 2007 and being awarded a PhD degree in 2008. He currently works as a senior clinical lecturer at the Zagreb Medical School and as a consultant internist at the University Hospital Zagreb and continues to have an active research interest into the effectiveness of medical education both at undergraduate and postgraduate levels while also conducting research in health outcomes measures, medical informatics as well as rational use of antibiotics. Another area in which Dr.Likic has had significant success is international collaboration with funding secured from the British Council to organize a multinational meeting in Zagreb in 2008 on rational use of medicines as well as getting the Matovinovic fellowship award in 2009 which allowed him to spend 6 months working as a visiting scholar at the University of Michigan Medical School (Ann Arbor). His interest in informatics led him to play a pivotal role in introduction of voice over internet protocol (VOIP) based telephony systems both at Zagreb and Ann Arbor medical schools that continue to provide means for inexpensive international telephony at both institutions. Dr.Likic is a member of several committees at the Zagreb Medical School as well as a member of the committee for education of the European Association for Clinical Pharmacology and Therapeutics (EACPT) and a councilor to the International Union of Basic and Clinical Pharmacology (IUPHAR). He received the “Outstanding Early Educator Award” at the 16th IUPHAR World congress of basic and clinical pharmacology held in 2010 in Copenhagen (Denmark).
Emilio Sanz obtained his MD degree (1981) and PhD (1983) in Medicine in the University of La Laguna and currently is professor in Clinical Pharmacology at the Medical School, responsible for the subjects of clinical pharmacology and prescribing. He has been the Dean of the medical school (2007-2015), when a major change in the educational curriculum was performed, and the Head of the Department of Pharmacology and Physical Medicine at the School of Health Sciences. He is also the Head Physician of the Clinical Pharmacology Service at the University Hospital of the Canary Islands, dealing with TDM and clinical consultations, as well as chair of the Ethics Committee for Clinical Research of the University Hospitals in Tenerife. Prof. Sanz is also the Director of the Interuniversity Master Programme in Bioethics and Biolaw. He has been involved in educational projects since the early 90’s and has published several papers in educational matters for teaching of prescribing. In July 2019 he was awarded with the “Lifetime achievement in Clinical Pharmacology Education” prize of the European Association for Clinical Pharmacology and Therapeutics.
Jelle Tichelaar is Assistant Professor and Education Coordinator of the section Pharmacotherapy, Department Internal Medicine at the VU University Medical Center in Amsterdam. Within the Education working group of the EACPT he is the professional secretary and chairman of the working group Research on Education. He is also board member of the education working group of both the Dutch Society of Clinical pharmacology and Bio pharmacy (NVKF&B) and the International Union of Basic and Clinical Pharmacology (IUPHAR).
He received the “Outstanding Early Educator Award” in the category Clinical pharmacology at the 17th IUPHAR World congress of basic and clinical pharmacology held in 2014 in Cape Town South Africa.
He developed and organized several Teach the Teacher courses for CPT teachers both nationally and internationally (EACPT Summer School on Education, Amsterdam 2012) and he is co-initiator of the Dutch National Pharmacotherapy Examination regarding medication safety.
Main research interest (Home – Recipe Amsterdam UMC (recipe-amsterdam.nl) ) :
1. The development of effective and attractive education for undergraduate and postgraduate students and teachers, based on knowledge of the process of therapeutic thinking and acting, as well as on recent insights in the field of education (evidence-based education)
2. Conducting research concerning the process of therapeutic thinking and acting of physicians and under-and postgraduate students, particularly the treatment and drug selection process, both theoretically (declarative research), as well as in relation to schooling and training (impact research).
João Costa receive is licensure in Medicine (School of Medicine, University of Lisbon) in 1998. He is medical specialist in neurology, clinical neurophysiologist and in clinical pharmacology. He received clinical research training in the Walton Centre for Neurology and Neurosurgery (WCNN) NHS Trust, Liverpool, UK and in the Neurology Department of the Hospital Clínic of Barcelona, Spain. Currently he practice medicine in Hospital da Luz, Lisbon, Portugal. After finishing his licensure, he got the following academic degrees and titles: Master of Neurosciences (University of Lisbon); Master of Neurological electrodiagnosis (University of Barcelona); and Doctor of Medicine, Discipline of Neurology (University of Lisbon). He had a Postdoctoral Research Fellowship by CIBERNED to work for a year in movement disorders at Hospital Clínic of Barcelona, Spain. His major current positions are: Professor of Clinical Pharmacology and Therapeutics at School of Medicine, University of Lisbon; Coordinator Editor of the Cochrane Movement Disorders Group; Coordinator Director of Cochrane Portugal; Principal Investigator at the Clinical Pharmacology Unit and at the Translational Clinical Physiology Unit of the Institute of Molecular Medicine (Lisbon, Portugal), Member of the Clinical Pharmacology College of the Portuguese General Medical Council, Member of the Medicines Evaluation Commission of the Portuguese Authority of Medicines and Health Products (Infarmed), and President of the Portuguese Clinical Neurophysiology Association. He is ad-hoc reviewer for 12 international journals. He has published 140 full papers in peer review journals. He presented 170 communications at scientific conferences. He has written 8 book chapters and 7 national clinical guidelines. He has received 10 Awards and Distinctions as first author/investigator by national and international institutions.
Details on scientific publications can be consulted at:
http://scholar.google.pt/citations?hl=pt-PT&user=1thYaC4AAAAJ&view_op=list_works&sortby=pubdate
Dr. R. Maciulaits was born on December 7th 1960 in Sovietsk, Kaliningrad region, Russia. He was trained as a pharmacist and as a medical doctor at the Lithuanian University of Health Sciences (LUHS) in Kaunas, Lithuania. Also in Kaunas he was trained in clinical pharmacology (PhD) and nephrology. He was appointed as assistant in clinical pharmacology at the LUHS in 1991, later combined with work in regulatory pharmacology and nephrology. His main areas of interest are clinical pharmacology teaching at both undergraduate (to medical, pharmacy students and nurses) and postgraduate levels (to residents of medical clinical pharmacology) and CP research in rational drug use, primarily antibioticotherapy and regulatory pharmacology. As the clinical and regulatory expert of the Lithuanian State Medicines Control Agency (SMCA) serves for scientific evaluations of national and European marketing authorizations, variations, referrals, and other clinically related procedures since 1992. He is in charge of National representation of regulatory and scientific competence in scientific committees at EMA since 2004 (member of the Committee for Medicinal Products for Human Use and since 2009 – Committee for Advanced therapies). He is a member of the national Council for Marketing Authorization since 2004 and chairs the Council since 2007. He contributed to number of public task forces: Ad hoc working groups for national legislation and implementation of EU law in medicines and bioethics (1994-2009), Efficacy Working Party (since 1998 (since 2011 – Rheumatology-Immunology WP)), nine (Co-) Rapporteurships for scientific evaluations of European centralized marketing authorization applications serving as an (Co-) Rapporteur and assessor for pharmacokinetic, pharmacodynamic, and clinical parts; five Rapporteurships or contributions to European guidance documents on development of medicinal products (Solid Organ Transplantation, Progression of Renal Failure, Cell Based Medicinal Products, Tissue Engineered Products, Stem Cell Therapies, Cartilage regeneration); Scientific Advises in Scientific Advise Working Party of the EMA and SMCA (eight scientific advises in biomarkers qualification procedures and medicines developments – in the areas of translational, clinical and regulatory nephrology, solid organ transplantation, cell therapies, and infectious diseases); Cell Based Working Party (2007-2012). He leads one European interuniversity Module in postgraduate pharmaceutical medicines development course (Cooperative European Medicines Development Course) that is co-sponsored by IMI/EC and serves as professor of clinical pharmacology and clinical pharmacy in LHSU. He is practicing nephrologist – serving to Kaunas LHSU Hospital Clinics. He has published more than 30 full papers in peer review journals. He contributed to more than 10 national clinical guidelines.
Thierry Christiaens was trained as General Practitioner and Clinical Pharmacologist (NVKF&B). He is assistant professor linked to the Heymans Institute of Pharmacology Ghent University, Belgium and director of the Clinical Pharmacology section. He is in charge of the training in clinical pharmacology and pharmacotherapy of the medical students at Ghent University. He gives also courses in the faculty of Pharmaceutical sciences, the Dentistry and in the Nursing department. Since 2008 he is editor in chief of the Belgian Centre of Pharmacotherapeutical Information (BCFI/CBIP), editing the Belgian Drug Formulary (Gecommentarieerd Geneesmiddelenrepertorium/ Répertoire Commenté des Médicaments) and the Folia Pharmacotherapeutica (a drug bulletin member of ISDB). He is co-author of nearly 50 A1 papers and 100 articles in Belgian journals in the field of pharmacotherapy.
Bogdan Tamba is Assoc. Profesor in Pharmacology and Algesiology at the University of Medicine and Pharmacy “Gr. T. Popa” Iasi, Romania and Co-founder & Head of Development of bioROne – the first biotech cluster in Romania.
He graduated medical school in Iasi in 2004 and got a board certification in Clinical Pharmacology and was awarded a PhD degree, both in 2010.
As a young researcher Dr. Tamba received an “Outstanding leadership award” from People to People International in 2006, was an EACPT bursary for the Summer School in 2008 and received a EACPT Young Investigator Bursary in 2009.
His experience include positions such as Clinical Pharmacologist at Grunenthal, Lead Project Scientist at “CEMEX” – advanced pre-clinical research centre (financed by the RO govt. and EU, totaling over EUR 10 mil. for 16 months), Principal Investigator at the Centre for the Study and Therapy of Pain, member or director of 16 research projects (7 international with multiple partners) totaling over €8 mil or President of the Organizing Committee of the Romanian National Pain Congresses in 2014 and 2015.
The main research areas are clinical pharmacology, drug development, nanotechnologies, pain, and inflammation and resulted in 2 books and over 80 published papers.
His interest in education of pharmacology spans over 12 years of teaching (graduate and post graduate), educational grants and initiatives and publications.
Dr. Tamba is a member of the EACPT, International Association for the Study of Pain (IASP), American College of Clinical Pharmacology (ACCP) and Treasurer of the Algesiology Association of Romania (AAR).
Lorena Dima is Assoc. Prof. of Pharmacology, Clinical Pharmacology, Methodology of Scientific Research and Bioethics at the Faculty of Medicine of TRANSILVANIA University of Brasov. She is medical doctor by profession, graduated The Faculty of Medicine of University of Medicine and Pharmacy “Carol Davila” Bucharest and specialized in Clinical Pharmacology. She defended her PhD Thesis in the Romanian Academy and was awarded the PhD degree in 2012.
She has been involved in academic work in the Faculty of Medicine since 2000 and a promoter of introducing the Clinical Pharmacology discipline as a distinct discipline in the study plan of medical students. She has been directly involved in the process of study recognition between European Faculties as Departmental Erasmus/LLL/Erasmus Plus Coordinator for the Faculty of Medicine (since 2003). Since 2015 Lorena Dima is Head of the Department of Fundamental, Prophylactic and Clinical Disciplines.
Lorena Dima has the experience of international collaboration activities, as member of International Research or Educational Grants, some of them large-scale, involving top international specialists, such as FP7 project PLANT food supplements: Levels of Intake, Benefit and Risk Assessment (PLANTLIBRA) 2010-2014. She has been director or member of national and international educational and research projects, member of organizing committees of scientific events and had many collaborations in writing scientific papers in top rated international journals.
The academic activity passionately dedicated to teaching pharmacology to medical students for 17 years with constant interest in improving it by making it more attractive to students and, consequently, more efficient involves the research on education, which is one of the areas of interest. Other research interest areas are psychopharmacology and pharmacology of plant compounds.
Lorena Dima is member of European Association for Clinical Pharmacology and Therapeutics (EACPT), British Pharmacological Society (BPS), European Behavioral Pharmacology Society, Medical Balkan Union.
Jamie Coleman is Professor of Clinical Pharmacology and Medical Education at the University of Birmingham, UK. A current British Pharmacological Society Clinical Section Member, Jamie enjoys a wide range of educational and research responsibilities in the UK. Jamie is Deputy Programme Director and Therapeutics Lead Teacher for the third largest Undergraduate medical degree in the UK, and also chairs the Assessment Board of the highly successful Prescribing Safety Assessment in the UK and Ireland. As a recognised National and International expert in electronic prescribing, he also inputs to national strategy in digital medicines management and is co-Principal Investigator on a large Research Programme investigating ePrescribing effects on medication safety in England. Other responsibilities include chairing the UK National Specialty Advisory Committee for doctors training in CPT and providing input to the pharmacovigilance expert advisory group and licensing committee meetings at the MHRA.